Angelica Cheng
Active Member

Permitting IVF polygenic testing is a slippery slope to genome editing in hyper-competitive Asian societies like Singapore
The Asia-Pacific region has seen rapid economic growth over the...
 bioedge.org
bioedge.org
Permitting IVF polygenic testing is a slippery slope to genome editing in hyper-competitive Asian societies like Singapore
The Asia-Pacific region has seen rapid economic growth over the past few decades, fueled in large part by hyper-competitive social norms and Confucian values of various East Asian societies that emphasize hard work, frugality and education. The phenomenon of “tiger parenting” is common and widespread in these Asian societies, with many parents competing to get their children into the best schools even before they are born, through expensive purchases of residential properties near prestigious elementary schools.
Nowhere is this more apparent than in Singapore, which has a prevalent “kiasu” (afraid to lose) mentality in society. In this small ultra-rich city state, parents often push their children into the educational rat race at a very young age, and typically spend a large proportion of their income on after-school tuition fees. The absurdity of “tiger parenting” has even extended to recreational and enrichment activities such as music, sports, and art, whereby parents try their best to get their children to participate in various competitions, to inculcate the principle of trying to excel in everything they do.
Nevertheless, the major confounding factor to any parent’s best-laid plans is the randomness and unpredictability of the natural process of fertilization that involves mixing and recombining genes from the sperm and egg to produce new genetic variants. This is often manifested by siblings born from the same set of parents differing so much in looks, health and academic ability. Hence, there is always the worrying possibility that a child born of high-achieving parents may not necessarily be a high-achiever; with the risk that all the effort and money invested in a “dud” child will completely go to waste.
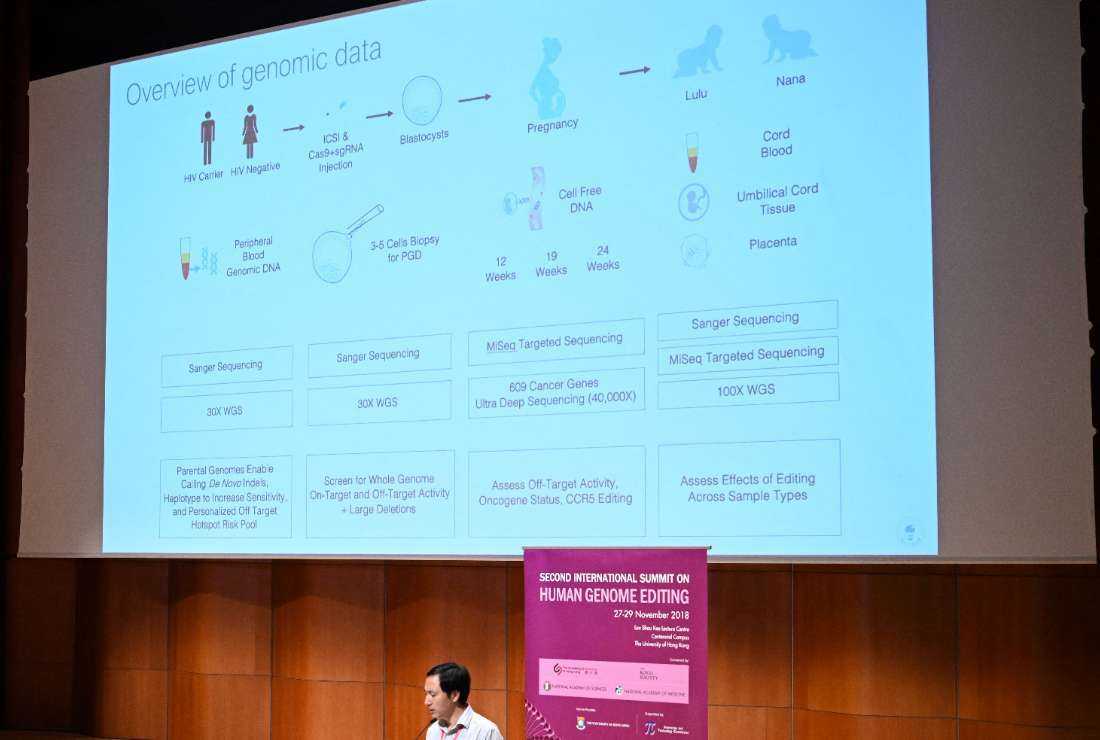
The advent of polygenic scoring in preimplantation genetic testing (PGT-P) of IVF embryos could possibly provide a solution to this confounding challenge. These can be applied both for the prevention of genetic diseases, as well as enhancement of socially-desirable traits such as intelligence, athletic ability and beauty standards linked to tallness, fair complexion, hair and eye colour.
Polygenic testing refers to evaluating an individual embryo’s likelihood of developing an adult-onset, multi-factorial trait by analyzing the combination of specific genetic variants within its genome.
For example, type II diabetes, obesity, intelligence, height, and skin complexion are such complex traits determined by the combination of multiple genes, so polygenic scores based on the presence of multiple gene variants are used to estimate the likelihood of developing such traits later in life. Currently, artificial intelligence (AI) algorithms are specifically being developed for such complex screening and analytical processes in polygenic testing. In a recent medical breakthrough, researchers in China used polygenic testing to select IVF embryos with less risks of developing family-related diabetes, for parents with a family history of the disease.
Unlike serious safety issues with human genome editing, there are minimal risks involved in polygenic testing and selection of IVF embryos, because there are no permanent man-made genetic modifications that would be passed down to future generations. It is basically a technique for picking the “winning ticket” in the “genetic lottery” of fertilization for good health, intelligence and other socially-desirable traits. Hence, this technique thus represents an attempt to overcome the unpredictability and randomness of the natural process of human fertilization to yield the best outcome.
This is a particularly lucrative business opportunity for fertility clinics, because parents naturally and instinctively want the best for their children, particularly in a hyper-competitive Asian society like Singapore. Indeed, a recent large-scale survey conducted in the US showed that 38% of respondents indicated that they would use polygenic testing to improve their child’s IQ and academic performance, to better their chances of entering an elite college. The corresponding figure would likely be much higher in Singapore.
Very likely, Singapore would ban polygenic testing of IVF embryos for selection of non-disease traits such as intelligence, while allowing its use for prevention of complex multi-factorial diseases such as type 2 diabetes.
However, this could possibly lead to a “slippery slope” situation, whereby the need to prevent common family-related disease traits such as type 2 diabetes can be readily exploited as a “convenient excuse” to also secretly select for non-disease socially-desirable traits such as intelligence, tallness and fair complexion.
The fact remains that the genomic DNA sequence of IVF embryos will likely be made available to patients who did polygenic testing for preventing diseases such as type 2 diabetes. It is difficult to deny such data to patients who pay for it, particularly for private DNA sequencing companies with overseas branches or headquarters. With just a few clicks, encrypted digital data anonymously labeled with QR or bar codes can be secretly transferred across the globe. These patients can first freeze their DNA-sequenced IVF embryos, and send such data overseas for prediction of intelligence, tallness and fair complexion, without any involvement of the fertility clinic or knowledge of their doctor.
Additionally, it is foreseen that the technical limitations of IVF polygenic testing would very soon lead to much disappointment and frustration among its many eager proponents, who expect to see vast improvements in their offspring after spending so much money.
For example, the statistical probability of two short parents conceiving a tall child by polygenic testing would always remain low, because most of the genes that predispose to tallness are simply absent in those parents. The same can be said of two average IQ parents attempting to conceive a high IQ child, or two dark-skinned parents attempting to have a fair-skinned baby by polygenic testing. In fact, because most selections occur between embryos with the same parents, this substantially limits genetic variability, and hence limits the usefulness of polygenic testing in targeting complex traits such as intelligence, tallness and fair skin. There is a much reduced number of possible outcomes, when selecting for specific characteristics within such a small sample size with limited variability, which obviates the usefulness of any selection method.
This would likely result in the disappointed and frustrated customers of IVF polygenic testing advocating for a better and more effective method to conceive their perfect “dream child”. That would most likely be human genome editing with all its risks and safety issues.
An appropriate analogy here would be a drug addict progressing from soft drugs such as cannabis, to hard drugs such as heroin and crack cocaine, because of temptation to be gratified by better and longer-lasting “highs”. Hence, permitting IVF polygenic testing would likely be a slippery slope to human genome editing in a hyper-competitive Asian society like Singapore.
Last edited: