Angelica Cheng
Active Member
Leading Experts Comment in Prestigious Medical Journal on 'The Uncertain Science of Preimplantation and Prenatal Genetic Testing'
Tests to identify chromosomal abnormalities in embryos and in early pregnancies rely on outdated science. Regulation of these genetic tests is urgently needed to ensure transparency and validation.
Leading Experts Comment in Prestigious Medical Journal on 'The Uncertain Science of Preimplantation and Prenatal Genetic Testing'
Tests to identify chromosomal abnormalities in embryos and in early pregnancies rely on outdated science. Regulation of these genetic tests is urgently needed to ensure transparency and validation.
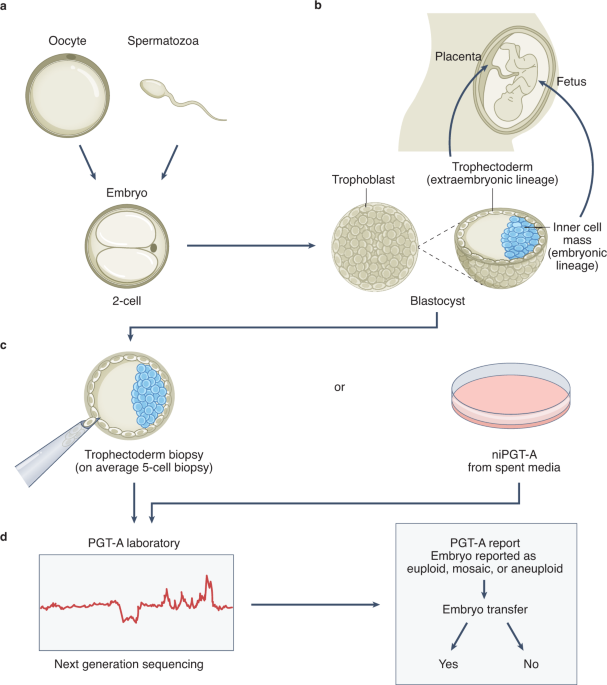
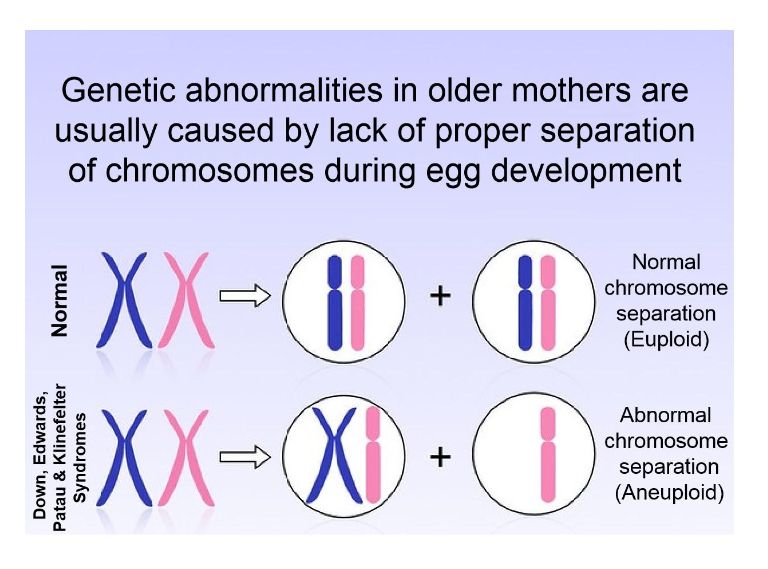
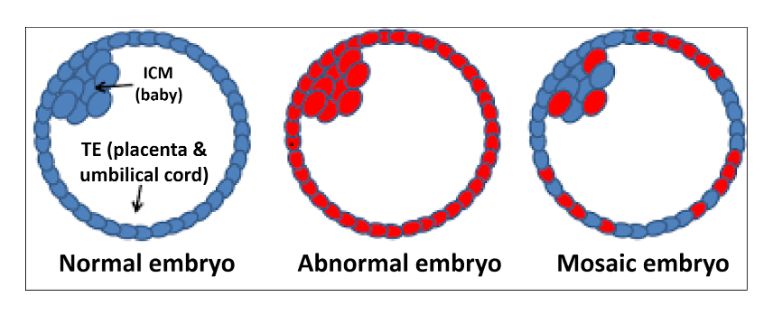
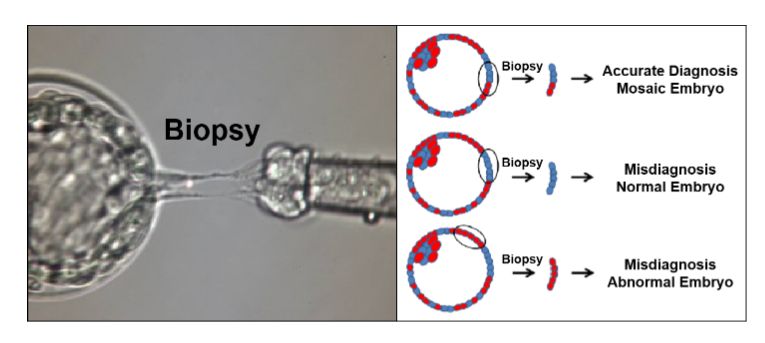
Five prominent scientists from the U.S. and Israel, including lead author Norbert Gleicher, founder and medical director of the Center for Human Reproduction, just published a commentary in the highly prestigious medical journal, NATURE Medicine, pointing to the shortcomings of current genetic testing methods and procedures in reproductive medicine. Preimplantation genetic testing of embryos for chromosomal abnormalities (aneuploidies), now given the term preimplantation genetic testing for aneuploid (PGT-A), has been used in association with in vitro fertilization (IVF) with increasing frequency since the 1990s. While the underlying testing technology has over the decades greatly improved, the utilization of PGT-A has remained highly controversial because the test never was able to establish clear benefits for IVF outcomes. In more recent years, moreover, increasing evidence has been developed suggesting that PGT-A in certain patient populations, indeed, may reduce pregnancy chances with IVF.
In parallel, the genetic testing industry has also been developing tests to discover chromosomal abnormalities in early pregnancy through maternal blood tests based on so-called cell-free DNA. Both testing premises have been criticized for high numbers of false-positive results, in association with PGT-A leading to disposal and/or non-use of huge numbers of embryos with excellent pregnancy and live birth potential, and in association with early prenatal diagnosis, to potentially terminations of perfectly normal pregnancies, as a recent article in The New York Times pointed out (Kliff S and Bhatia A, January 1, 2022). The genetic testing industry in association with some IVF centers, moreover, recently also started commercial offerings of so-called polygenic risk scoring of human embryos, where the purpose no longer is to detect chromosomal abnormalities or embryos affected by single-gene diseases, but polygenic traits, like eye color, specific physical traits and/or talents and polygenically transmitted diseases, a process the European Society of Human Genetics (ESHG) just weeks ago describes as "unproven and unethical."
The authors also comment on the fact that these tests, as so-called laboratory-developed tests, under a loophole in current legislation that originally was meant to help small doctor-office laboratories, do not fall under FDA supervision and, therefore, can be brought to market with no or only inadequate prior validation studies. Poorly or completely unvalidated diagnostic tests must, however, be considered "experimental" and, therefore, should only be offered in clinical practice under research protocols until appropriate validation studies confirmed their alleged benefits. The authors note, furthermore, that professional organizations in the field have so far failed to intervene and urge them to do so before government agencies step in.
Norbert Gleicher, M.D., the lead author of this publication, is available for further comments by calling (212) 994-4400 or e-mailing [email protected]. Those contacts can also make available the other four authors of the manuscript. Norbert Gleicher, M.D., is the Medical Director and Chief Scientists of The Center for Human Reproduction in New York City, a leading infertility center. He is also a Visiting Research Scientist at Rockefeller University in New York City and a Professor (Adj.) in the Department of Obstetrics and Gynecology at the Medical University Vienna in Vienna, Austria.