Angelica Cheng
Active Member
Stringent criteria needed for germline genome editing of human IVF embryos - PubMed (nih.gov)
Chin AHB, Nguma JB, Ahmad MF. Stringent criteria needed for germline genome editing of human IVF embryos. 2024 May 2. J Assist Reprod Genet. doi: 10.1007/s10815-024-03125-6.
Abstract:
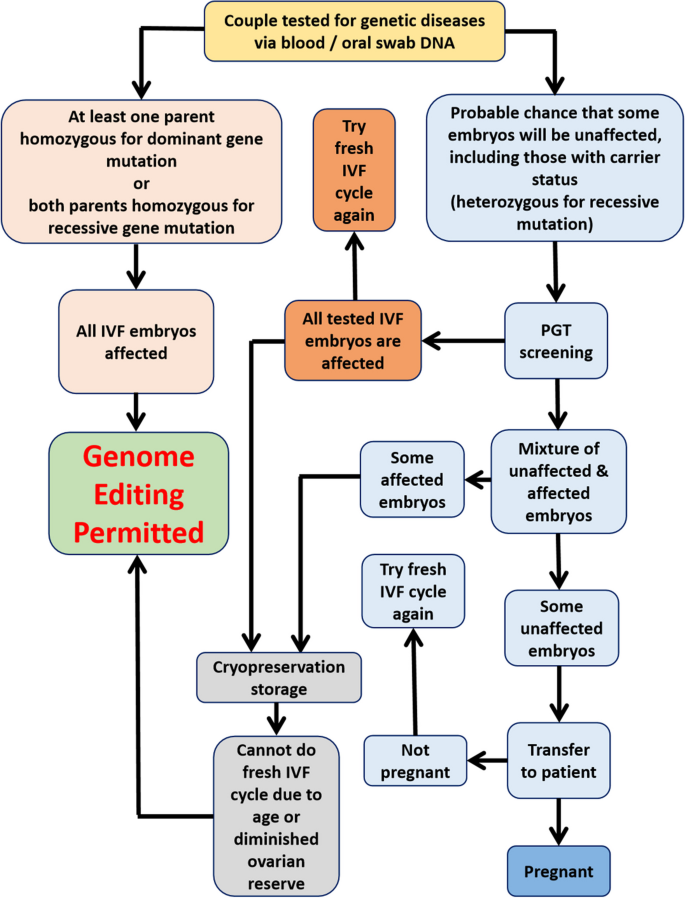
Germline genome editing of IVF embryos is controversial because it is not directly health or lifesaving but is intended to prevent genetic diseases in yet-unborn future offspring. The following criteria are thus proposed for future clinical trials: (i) Due to medical risks, there should be cautious and judicious application while avoiding any non-essential usage, with rigorous patient counseling. (ii) Genome editing should only be performed on the entire batch of IVF embryos without initial PGT screening if all of them are expected to be affected by genetic disease. (iii) When there is a fair chance that some IVF embryos will not be affected by genetic diseases, initial PGT screening must be performed to identify unaffected embryos for transfer. (iv) IVF embryos with carrier status should not undergo germline genome editing. (v) If patients fail to conceive after the transfer of unaffected embryos, they should undergo another fresh IVF cycle rather than opt for genome editing of their remaining affected embryos. (vi) Only if the patient is unable to produce any more unaffected embryos in a fresh IVF cycle due to advanced maternal age or diminished ovarian reserves, can the genome editing of remaining affected embryos be permitted as a last resort.
Keywords: CRISPR-Cas9; Ethics; Gene therapy; Monogenic disorders; Patient enrolment; Preimplantation embryo
Flowchart illustrating proposed criteria for enrolling patients in future clinical trials of germline genome editing of IVF embryos
Chin AHB, Nguma JB, Ahmad MF. Stringent criteria needed for germline genome editing of human IVF embryos. 2024 May 2. J Assist Reprod Genet. doi: 10.1007/s10815-024-03125-6.
Abstract:
Germline genome editing of IVF embryos is controversial because it is not directly health or lifesaving but is intended to prevent genetic diseases in yet-unborn future offspring. The following criteria are thus proposed for future clinical trials: (i) Due to medical risks, there should be cautious and judicious application while avoiding any non-essential usage, with rigorous patient counseling. (ii) Genome editing should only be performed on the entire batch of IVF embryos without initial PGT screening if all of them are expected to be affected by genetic disease. (iii) When there is a fair chance that some IVF embryos will not be affected by genetic diseases, initial PGT screening must be performed to identify unaffected embryos for transfer. (iv) IVF embryos with carrier status should not undergo germline genome editing. (v) If patients fail to conceive after the transfer of unaffected embryos, they should undergo another fresh IVF cycle rather than opt for genome editing of their remaining affected embryos. (vi) Only if the patient is unable to produce any more unaffected embryos in a fresh IVF cycle due to advanced maternal age or diminished ovarian reserves, can the genome editing of remaining affected embryos be permitted as a last resort.
Keywords: CRISPR-Cas9; Ethics; Gene therapy; Monogenic disorders; Patient enrolment; Preimplantation embryo
Flowchart illustrating proposed criteria for enrolling patients in future clinical trials of germline genome editing of IVF embryos

