Angelica Cheng
Active Member

Hidden in plain sight: the overstated benefits and underestimated losses of potential implantations associated with advertised PGT-A success rates
Abstract. The utilization of preimplantation genetic testing for aneuploidy (PGT-A) has understandable intuitive appeal in reassuring the clinician that ‘e
Hidden in plain sight: the overstated benefits and underestimated losses of potential implantations associated with advertised PGT-A success rates
Abstract
The utilization of preimplantation genetic testing for aneuploidy (PGT-A) has understandable intuitive appeal in reassuring the clinician that ‘everything possible’ has been done to assure the birth of a healthy baby. Whereas the development of the PGT-A technology is still in a relatively early stage, great strides have nevertheless been made in the understanding of the genetics of the preimplantation human embryo. The problem lies not in the progress that has been achieved, but rather, in the reality that PGT-A is being actively marketed as a mature technology. Those that market the technology overstate its benefits and underestimate the losses of potential implantations that are the consequence of the practice of PGT-A. The implication is that the PGT-A technology is accurate, has minimal errors and is ready to be applied to every case of IVF. This approach is not evidence-based. Substantial losses of potential implantations are even evident in the analysis of the numbers presented by marketing materials themselves. In order to provide accurate, evidence-based counseling for patients undergoing IVF, we need to apply an appropriate level of scientific scrutiny to the data that are available and apply PGT-A selectively to those cases in which the benefits clearly outweigh the costs.
Introduction
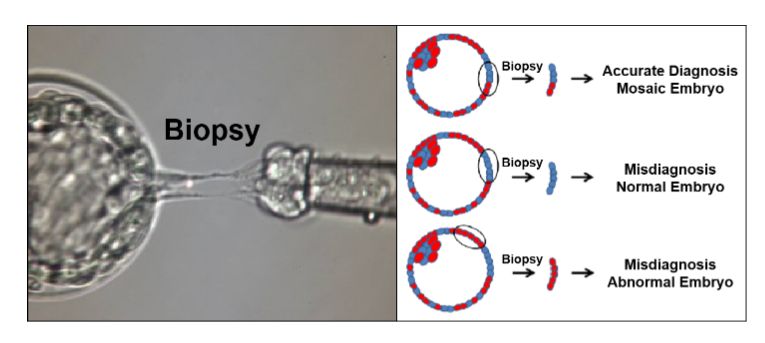
It is easy to appreciate the intuitive appeal of preimplantation genetic testing for aneuploidy (PGT-A) in helping all of us achieve our common goal of a healthy baby after IVF. Patients as well as clinicians want to maximize the probability of a successful pregnancy following embryo transfer (ET), while minimizing the risk of multiple gestations. There is also the satisfaction of feeling that one has done ‘everything possible’ to minimize the disappointment of a failed ET, miscarriage or even a pregnancy termination triggered by a chromosomally abnormal pregnancy. Genetics and genomics play an increasingly important role in our field, and genetic analysis of the preimplantation human embryo, especially if it can be achieved without the trauma of an embryo biopsy (Ho et al., 2018), may provide important information about the biology of human preimplantation development.
Marketing PGT-A
The problem with the current clinical application of PGT-A is that its use has become highly marketed. Commercial ventures are selling this technology, which is still quite early in its development, as a mature, established diagnostic technique. As a consequence, clinicians are presented by marketing rather than carefully scrutinized scientific data. Those that market PGT-A want to see it applied to every case of IVF and are understandably motivated to overstate the magnitude of the anticipated benefits. For example, advertised numbers (Igenomix, n.d.) suggest that ongoing embryo implantation rates (implantations leading to a live birth or successful pregnancy) in women younger than 35 years increase from 49.4% to 65% when blastocysts are transferred without and with, respectively, the prior use of PGT-A. These numbers contrast with the published results of Kang et al. (2016), who reported unchanged ongoing embryo implantation rates (of ~50%) in women younger than 35 years whose blastocysts did or did not undergo testing with PGT-A. The advertised numbers also contrast with those reported in the STAR trial (Illumina, 2019), a multi-center randomized clinical trial, which sought to demonstrate superior implantation rates in the PGT-A group, but which actually demonstrated similar ongoing implantation rates of ~50% in both study and control groups among women younger than 35 years. Both of the latter studies confirmed that the ongoing implantation rates of ~50% observed with PGT-A in women older than 35 years were higher than the declining ongoing implantation rates observed in the controls. However, implantation rates of higher than 60% were not observed.
Implantation rates in untested embryos, PGT-A tested embryos and the aneuploidy rates as presented in the patient brochure available at genomix.com*
Table I
Implantation rates in untested embryos, PGT-A tested embryos and the aneuploidy rates as presented in the patient brochure available at genomix.com*.
| Age (years) | Untested implantation rate | PGT-A tested implantation rate | Aneuploidy rate |
|---|---|---|---|
| <35 | 49.4% | 65.0% | 51.8% |
| 35–37 | 42.3% | 64.5% | 54.4% |
| 38–40 | 32.9% | 61.1% | 67.9% |
| 41–42 | 20.7% | 60.2% | 77.9% |
| >42 | 7.8% | 53.7% | 79.8% |
*Available at: https://www.igenomix.us/hubfs/USA/PGS/PDF/PGS patient trifold US.pdf. Accessed 10 June 2019.
At the same time, those that seek to market the PGT-A technology substantially underestimate the magnitude of losses associated with PGT-A. For example, a recent publication proposing the cost-effectiveness of PGT-A estimated that ‘PGT-A is associated with a 5% relative reduction in live birth rate due to the theoretical biopsy-related damage to the embryo and to the false-positive results that may derive from the inherent technical error of chromosomal diagnostics’ (Somigliana et al., 2019). This very low estimate of 5% is in direct conflict not only with scientific publications, but also with actual advertised numbers. However, the advertised numbers must be analyzed to reveal this discrepancy, and that is the purpose of the following analysis.
PGT-A as a purification procedure
The method by which PGT-A seeks to increase implantation rates of transferred embryos is not by improving the quality of the embryos but rather by eliminating from the cohort of embryos those that are less likely to implant. This can be thought of as a purification procedure, such as one that might be applied to the purification of a specific chemical from a mixture of chemicals or to gold being extracted from gold ore. In all these cases, in order to increase the purity of a desired substance from an impure mixture of substances, some part of the original sample is discarded with the intent that the remaining sample will have a higher concentration of the desired substance. Since purification procedures are not perfect, each purification method is necessarily associated with unintended losses of some of the desired substance. The amount of the desired substance that remains in the sample is reflective of the efficiency of the purification. In the case of PGT-A, the desired substance is ‘embryos capable of implantation’ and the ‘purity’ of the substance is the ‘implantation rate of the embryos in the cohort.’ Embryos that are judged by PGT-A to be ‘aneuploid’ are discarded from the cohort, with the intent of increasing the implantation rate of the remaining embryos. The fact that some ‘aneuploid’ embryos have been found to implant (Patrizio et al., 2019) provides evidence that the selection is not perfect. This is analogous to discarding some gold from the purification of gold ore. In the case of PGT-A, there is a second possible mechanism for the loss of potential implantations, because biopsied embryos that are classified as ‘euploid’ may have decreased implantation rates due to the trauma of the embryo biopsy. Thus, discarding normal embryos and decreasing the implantation rate of the remaining embryos are two separate effects, which combine to produce losses of potential implantations. Their individual contributions cannot be calculated with the available data, but it is possible to calculate the total losses.